At 13 letters long Rutherfordium is the longest element name on the periodic table. Rutherfordium, 267Rf (element 104), is an artificial radioactive trans-uranium element with an electronic configuration [Rn] 5f14 6d2 7s2. It was named after renowned nuclear physicist Ernest Rutherford (a New Zealand-born British scientist). [1]
Discovery
The credit for the discovery of element 104 remained disputed between Soviet (Russian) and American scientists. In 1964, a team of Russian researchers headed by George Flerov at the Joint Institute for Nuclear Research (JINR) located in Dubna first claimed the discovery of the element 104 and named the element as Kurchatovium (Ku) after the name of Igor Vasilyevich Kurchatov, a Soviet nuclear physicist.
They Bombarded Plutonium-242 with a beam of Neon-22 ions and observed nuclear fission which predicted the presence of a new element. This discovery was not accepted universally but repeated experiments in 1966 in Dubna confirmed the previous discovery. On the basis of calculation, they predicted that possibly it was Rutherfordium 260. The reported half-life (t1/2) of the element was 0.3s which they change to 0.15s later on.[2]
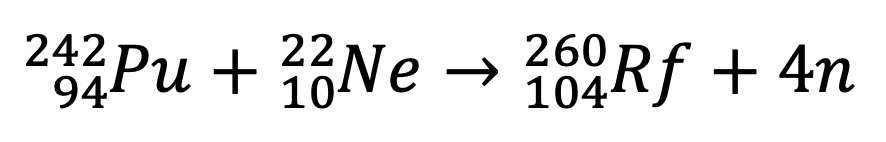
In 1969, a team of American scientists supervised by Albert Ghiorso at Californian Lawrence Radiation Laboratory at Berkeley attempted to get the same isotope but they used a different method. They bombarded californium-249 with Carbon-12 and Carbon-13 and obtained two different isotopes of element 104 with mass numbers 257 and 258 respectively. They also obtained an isotope with mass number 261 by bombarding curium-248 with oxygen-18. They proposed the name Rutherfordium in the honor of the name of Ernst Rutherford to element 104. [1, 3]
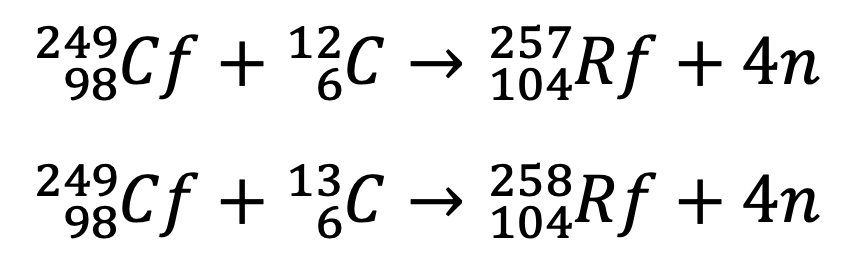
Controversy in naming
There has been a controversy between Soviet and American scientists in naming the element 104. It was temporarily called Unnilquadium (Unq) by the “International Union of Pure and Applied Chemistry” (IUPAC).[4] IUPAC decided to credit both Dubna and Berkeley teams but the latter was given more credit because they were able to synthesize a large number of 257 and 258 isotopes of Rutherfordium having longer half-lives and were able to detect the decayed product (Nobelium isotope) while Dubna team was able to synthesize only a few atoms of Rf-260. Therefore, IUPAC finalized the name “Rutherfordium” for this element in 1997.[5] Dubna team was credited by giving the name dubnium (Db) to element 105. [4]
Isotopes
Rutherfordium is a synthesized radioactive element comprising 16 unstable radioisotopes having atomic mass from 253-270 (266Rf, 268Rf, and 270Rf are unconfirmed) while 267Rf is the most stable of all with ~1.3h of t1/2. [6]
Properties
It is the first transactinide radioactive element synthesized chemically in the laboratory either by fusion of atoms or disintegration of heavy isotopes. It is a d-block transition metal from Group IVB and Period 7 of the periodic table. It is predicted to exist as solid-state metal and shows similarity with hafnium.[7]
Application
It has no practical application in daily life but is very important for research purposes.[3]
Protactinium
At 12 letters long, Protactinium is tied for the second longest element name on the periodic table. Protactinium (91Pr) is a heavy, shiny, and silver-gray radioactive actinoid first predicted by Dmitri Mendeleev in 1871. In 1900, William crooks isolated it from Uranium and named it Uranium-X but he didn’t specify it as a new element. Its first short-lived isotope 234Pa was discovered by Kasimir Fujan and Otto Gohring in 1913 and was named Brevium meaning ‘brief’ due to its short half-life (6.7h) while long-lived isotope 231Pa with a half-life of 32,760 years was discovered by Otto Hahn from Germany and Lisa Meitner from Australia, and independently by British scientist Frederick Soddy and John Cranston in 1918 and was called protoactinium.
In 1934, Aristid V. Grosse first isolated it. In 1949, IUPAC shortened the name to protactinium which means Parent of actinium because its radioactive decay produces actinium. Due to radioactivity, it is used only for research reasons. [8,9]
Praseodymium
Praseodymium is another long element name, measuring 12 letters in length. Praseodymium (59Pr), is a soft, malleable, and silvery metal, placed at the third position in the Lanthanide series. A chemist from Austria, “Carl Welsbach” discovered it together with Neodymium (Nd) from a lanthanoid mixture “Didymium”(Di) in 1885. It was named using Greek vocables ‘prasios (green) didymos (twin)’ collectively called ‘green twin’ because of its pale green salts and lanthanum-like behavior. As an alloying agent, it is widely used in Mischmetal, plane engines, C arc electrodes, and welder spectacles. [10]
Darmstadtium
The element Darmstadtium is another of the three elements tied for second place as the longest element name on the periodic table. Darmstadtium (110Ds), is an intensely radioactive, synthetic element of d-block that was synthesized by a group of international scientists from GSI in Darmstadt and was named after the city of Darmstadt where it was discovered. Its properties are still unknown because of its fast decay rate, high cost, and small production, and is used in fundamental scientific research. [11,12]
Conclusion
Rutherfordium is the longest element name measuring 13 letters long, followed by Protactinium, Praseodymium, and Darmstadtium all measuring 12 letters in length. As always we used the character counter on our home page to measure the name lengths. We hope you enjoyed this article!
References:
1) Rutherfordium – Element information, properties, and uses | Periodic Table. Rsc.org. (2022). Retrieved 25 June 2022, from https://www.rsc.org/periodic-table/element/104/rutherfordium.
2) National Center for Biotechnology Information (2022). PubChem Element Summary for Atomic Number 104, Rutherfordium. Retrieved June 24, 2022, from https://pubchem.ncbi.nlm.nih.gov/element/Rutherfordium.
3) Stewart, Doug. (2012, October 24). Rutherfordium. Rutherfordium; www.chemicool.com. https://www.chemicool.com/elements/rutherfordium.html.
4) (1997). Names and symbols of transfermium elements (IUPAC Recommendations 1997). Pure and Applied Chemistry, 69(12), 2471-2474. https://doi.org/10.1351/pac199769122471
5) Britannica, T. Editors of Encyclopaedia (2021, May 16). rutherfordium. Encyclopedia Britannica. https://www.britannica.com/science/rutherfordium.
6) Isotopes of rutherfordium – Wikipedia. (2008, June 6). Isotopes of Rutherfordium – Wikipedia; en.wikipedia.org. https://en.wikipedia.org/wiki/Isotopes_of_rutherfordium.
7) Rutherfordium – Element information, properties, and uses | Periodic Table. Rsc.org. (2022). Retrieved 25 June 2022, from https://www.rsc.org/periodic-table/element/104/rutherfordium.
8) National Center for Biotechnology Information (2022). PubChem Element Summary for Atomic Number 91, Protactinium. Retrieved June 25, 2022, from https://pubchem.ncbi.nlm.nih.gov/element/Protactinium
9) Morss, L. (2019, November 21). protactinium. Encyclopedia Britannica. https://www.britannica.com/science/protactinium.
10) National Center for Biotechnology Information (2022). PubChem Element Summary for AtomicNumber 59, Praseodymium. Retrieved June 25, 2022 from https://pubchem.ncbi.nlm.nih.gov/element/Praseodymium.
11) National Center for Biotechnology Information (2022). PubChem Element Summary for AtomicNumber 110, Darmstadtium. Retrieved June 25, 2022 from https://pubchem.ncbi.nlm.nih.gov/element/Darmstadtium.
12) Darmstadtium – Wikipedia. (2019, September 1). Darmstadtium – Wikipedia; en.wikipedia.org. https://en.wikipedia.org/wiki/Darmstadtium.